) with no science background will dismiss this as 'googling', but perhaps he should try some 'googling' instead of trying to argue using platitudes and fallacies and mere child-like dismissals of that which he cannot comprehend - if you need help with the relevant science terms, don't be ashamed to ask (be ashamed to try to argue against it all with mere dismissals or referrals to tribal deities):
Unified reaction pathways for the prebiotic formation of RNA and DNA nucleobases†
Yassin Aweis Jeilani,*
a Phoenix N. Williams,
a Sofia Walton
a and Minh Tho Nguyen*
b
Abstract
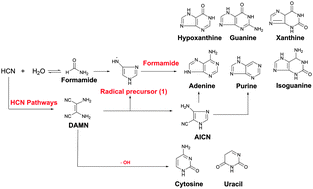
The reaction pathways for the prebiotic formation of nucleobases are complex and lead to the formation of a mixture of products. In the past 50 years, there has been a concerted effort for identifying a unified mechanism for the abiotic origin of the biomolecules but with little success. In the present theoretical study, we identified two prominent precursors for the building up of RNA and DNA nucleobases under prebiotic conditions: (a) 1,2-diaminomaleonitrile (DAMN), which is a tetramer of hydrogen cyanide (HCN), and (b) formamide, a hydrolysis product of HCN; it is important to emphasize that HCN is the source of both precursors. We find that free radical pathways are potentially appropriate to account for the origin of nucleobases from HCN. The current study unites the formamide pathways with the DAMN pathways. The mechanisms for the formation of the RNA and DNA nucleobases (uracil, adenine, purine, cytosine) were studied by quantum chemical computations using density functional theory at the B3LYP/6-311G(d,p) level. All the routes involved proceed with relatively low energy barriers (within the error margin of DFT methods). We showed that the radical mechanisms for the formation of nucleobases could be unified through common precursors. The results demonstrated that 4-aminoimidazole-5-carbonitrile (AICN), which is a known precursor for nucleobases, is a product of DAMN. The overall mechanisms are internally consistent with the abiotic formation of the nucleobases, namely (a) under a meteoritic impact scenario on the early Earth's surface that generated high internal energy, and/or (b) in the (gas phase) interstellar regions without the presence of catalysts.
Generation of RNA Molecules by a Base-Catalysed Click-Like Reaction
Dr. Giovanna Costanzo,Prof. Raffaele Saladino,Dr. Giorgia Botta,Dr. Alessandra Giorgi,Dr. Anita Scipioni,Dr. Samanta Pino,Prof. Ernesto Di Mauro,
First published: 30 March 2012
Abstract
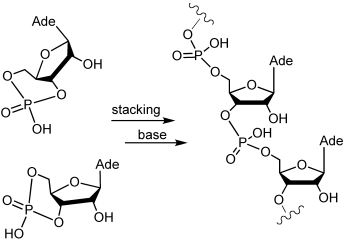
Spontaneous polymerization of 3′,5′-cyclic GMP occurs in water, in formamide, in dimethylformamide, and (in water) in the presence of a Brønsted base such as 1,8-diazabicycloundec-7-ene. The reaction is thermodynamically favoured and selectively yields 3′,5′-bonded ribopolymers.
 Abstract
Abstract
The problem of the abiotic origin of RNA from prebiotically plausible compounds remains unsolved. As a potential partial solution, we report the spontaneous polymerization of 3′,5′-cyclic GMP in water, in formamide, in dimethylformamide, and (in water) in the presence of a Brønsted base such as 1,8-diazabicycloundec-7-ene. The reaction is untemplated, does not require enzymatic activities, is thermodynamically favoured and selectively yields 3′,5′-bonded ribopolymers containing as many as 25 nucleotides. We propose a reaction pathway on the basis of 1) the measured stacking of the 3′,5′-cyclic monomers, 2) the activation by Brønsted bases, 3) the determination (by MALDI-TOF mass spectrometry, by 31P NMR, and by specific ribonucleases) of the molecular species produced. The reaction pathway has several of the attributes of a click-like reaction.
Abiotic synthesis of purine and pyrimidine ribonucleosides in aqueous microdroplets
Inho Nam, Hong Gil Nam, and Richard N. Zare
See all authors and affiliations
PNAS January 2, 2018 115 (1) 36-40; first published December 18, 2017;
Abiotic synthesis of purine and pyrimidine ribonucleosides in aqueous microdroplets
Significance
Discovery of an improved prebiotic method for the synthesis of ribonucleosides provides support to theories that posit a central role for RNA in the origin of life. It has been assumed that ribonucleosides arose through an abiotic process in which ribose and nucleobases became conjoined, but the direct condensation of nucleobases with ribose to give ribonucleosides in bulk solution is thermodynamically uphill. We show a general synthetic path for ribonucleosides, both purine and pyrimidine bases, using an abiotic salvage pathway in a microdroplet environment with divalent magnesium ion (Mg2+) as a catalyst. Purine and pyrimidine ribonucleosides are formed simultaneously under the same conditions, which suggests a possible scenario for the spontaneous production of random ribonucleosides necessary to generate various types of primitive RNA.
Abstract
Aqueous microdroplets (<1.3 µm in diameter on average) containing 15 mM D-ribose, 15 mM phosphoric acid, and 5 mM of a nucleobase (uracil, adenine, cytosine, or hypoxanthine) are electrosprayed from a capillary at +5 kV into a mass spectrometer at room temperature and 1 atm pressure with 3 mM divalent magnesium ion (Mg2+) as a catalyst. Mass spectra show the formation of ribonucleosides that comprise a four-letter alphabet of RNA with a yield of 2.5% of uridine (U), 2.5% of adenosine (A), 0.7% of cytidine (C), and 1.7% of inosine (I) during the flight time of ∼50 µs. In the case of uridine, no catalyst is required. An aqueous solution containing guanine cannot be generated under the same conditions given the extreme insolubility of guanine in water. However, inosine can base pair with cytidine and thus substitute for guanosine. Thus, a full set of ribonucleosides to generate the purine–pyrimidine base pairs A-U and I-C are spontaneously generated in aqueous microdroplets under similar mild conditions.
Synthesis of pyrimidines and triazines in ice: implications for the prebiotic chemistry of nucleobases.
Abstract
Herein, we report the efficient synthesis of RNA bases and functionalized s-triazines from 0.1 M urea solutions in water after subjection to freeze-thaw cycles for three weeks. The icy solution was under a reductive, methane-based atmosphere, which was subjected to spark discharges as an energy source for the first 72 h of the experiment. Analysis of the products indicates the synthesis of the s-triazines cyanuric acid, ammeline, ammelide, and melamine, the pyrimidines cytosine, uracil, and 2,4-diaminopyrimidine, and the purine adenine. An experiment performed as a control at room temperature, with the urea solution in the liquid phase and with the same atmosphere and energy source, led to the synthesis of hydantoins and insoluble tholin, but there was no evidence of the synthesis of pyrimidines or triazines. The synthesis of pyrimidines from urea is possible under a methane/nitrogen atmosphere only at low temperature, in the solid phase. The generation of both pyrimidines and triazines in comparable yields from urea, together with a possible role for triazines as alternative nucleobases, opens new perspectives on the prebiotic chemistry of informational polymers.
Just a sampling of the 2,850 returns I got when using Google Scholar, searching for 'abiotic generation of nucleobases'. Let's say half of them are irrelevant or redundant. That leaves 1400 research papers on abiotic synthesis of nucleobases. It took me far longer to copy paste the abstracts that it did to do the search - something you could do rather than smugly asserting we have no idea how these things came to be without Yahweh magic - for Whom's very existence there exists no evidence whatsoever (I do not consider hillbillies seeing Jesus' face on burnt toast proof, sorry).
And then there are the spontaneous generation of nucleotides... in space:
While even the most disingenuous, desperate, dishonest hack of a creationist knows that abiogenesis is irrelevant to evolution, at least we can point to people actually trying to understand things. Whence the evidence or research for 'dust to Adam' God magic? Which creationist organization is sponsoring or engaging in research on the matter?
Slavish devotion to ancient tales and the deity described therein (but nowhere else) is cute, but not a very substantive or fruitful way for adults in the 21st century to live their lives.


