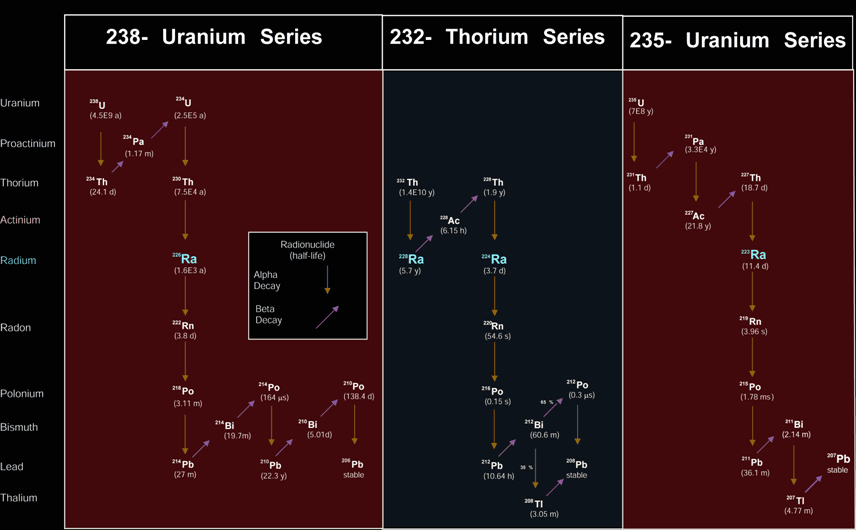
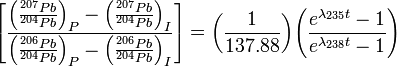You cannot possibly know if uranium, or any other isotope for that matter, has been affected by water or heat or leeched to give a closed system. You cannot possibly know what the initial content of lead in the initial rock sample was.
You are wrong, geochemists/geochronologists can detect those things and quantify them. And the initial amount of lead in a sample is totally irrelevant with today's technology, instrumentation and techniques. Have you been immersing yourself in the 'young earth proponents' literature? One of their tools of deception is to make reference to antiquated dating methods that are no longer in use and present them as if they were.
Now worst of all, and the icing on the cake, is that decay rates are not constant in sm146 and you have an average uranium ratio that differs remarkably. Now you cannot say decay rates are stable. You have proof in at least one shorter lived isotope that this is not the case.
Yep, create doubt where there actually is none. Why do you insist upon making erroneous statements? 146Sm is an "extinct" radionuclide. It does not exist naturally in this solar system. Extinct radionuclides have specific applications. Being an extinct radionuclide, its decay rate cannot be directly measured. In the past it was determined mathematically based on the known properties of radionuclides. However, recently scientists have been able to synthesize 146Sm in a reactor so the decay rate and other properties could actually be physically measured. WOW! Science advances and you consider it going backwards.
Upvote
0