Totally false. All we observe is that the isotopes NOW decay and do so at a certain rate. That says nothing about how it was at all.
The ratios of isotopes in rocks do tell us how it was in the past.
Get real. It makes for a better debate.
I am saying the actual isotopes, no matter how we chose to graph them existed, except for the stuff that decayed in this state.
Since you refuse to answer the question, I will answer it for you. The answer is no. A different state past would not produce rocks that would fall on that line in the graph because there is no reason that different rates of decay would produce rocks with isotopes that fall on the line in that graph.
However, a same state past would produce rocks that have ratios of isotopes that do fall on that line.
Therefore, we can measure the ratio of isotopes in rocks and see if they fall on that line or not. When we do those measurements, we find that they do fall on that line, as shown by this figure.
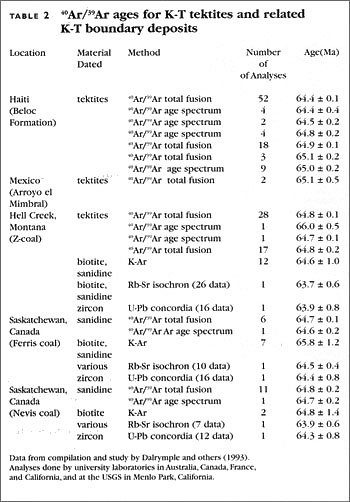
Therefore, we can prove that there was a same state past. We have also disproven a different state past.
Upvote
0

