But then there's some truly random events in QM which render definite mathematical descriptions of reality impossible in principle, right? The best that can be achieved are probabilities.
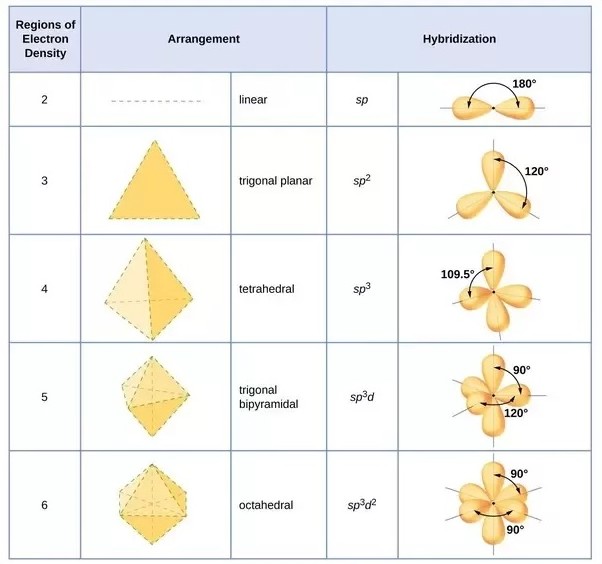
No the probabilistic nature of QM changed our notion of an electron from a particle to an electron density distribution or wave-particle duality characterized by its energy level, orbital momentum and orientation of the orbital momentum according to the quantum numbers n, l and mₗ respectively.
The electrons exist in
orbitals in an atom and are defined by the letters s, p, d, f... which depend on their quantum number combinations (n, l, mₗ).
The electron density distribution is as realistic as the electron was once treated as a classical particle.
The orbitals can mix or hybridize to various degrees which explains why molecules have the shapes they do and can only occur when the electron is seen as a density distribution rather than a particle.
Sounds like the complex perturbation is just "too hard" for mathematics, but not impossible in principle.
This is where mathematical modeling comes into the picture.
Quantum electrodynamics is a QFT (not QM) and based on electrons or particles being an excitation of the field and are point sources.
This is a mathematical not a physical description.
The interaction of two electrons causes serious problems at very small scales due to the electrons being point sources.
At these scales the momentum of the electrons is predicted to be infinite which is of course nonsense.
The solution is to ignore the mathematics at these small scales.
This is known as renormalization and makes quantum electrodynamics the most accurate theory known in physics.
On the surface this appears as Richard Feynman puts it mathematical hocus-pocus; to a pure mathematician it amounts to chopping up the mathematics to get the answer you are looking for.
There is however a physical reality to this.
As the electrons get close there is resonance or smearing effect where the electrons no longer behave as point sources but have a finite size.
Since electrons cannot get any closer due to this finite size, the small scales can be effectively ignored.
In this case complex perturbation will always remain “too hard” from a purely mathematical perspective but can be simplified by incorporating real effects into the mathematics.
This is how mathematical modeling is done as opposed to the rigorous methods of pure mathematics.