While life forms that are highly different from what we mostly know are fun and I'd love us to find life forms using other chemistry such as zero water and so on... (that would be great), it turns out that objectively (if you don't have some prejudice) water has some remarkable unusual qualities that are most useful, and which
you might not guess....
Some excerpts on how water has more than just 1 or 2 helpful effects, making it one of the possible useful aids to potential life forms (among various helpful chemistry, water stands out as exceptional).
And it's about everywhere too, let me add... Because of how supernovae make so much oxygen. So, it's not only having some useful attributes as you can read below, but also is very abundant. That doesn't hurt.
---------------
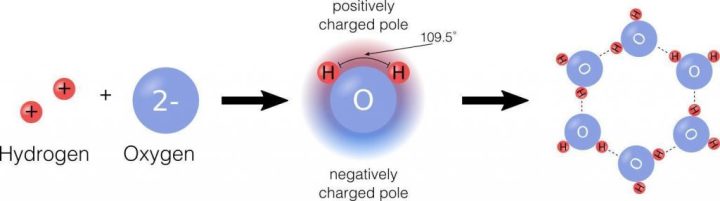
Many of water’s roles in supporting life are due to its molecular structure and a few special properties. Water is a simple molecule composed of two small, positively charged hydrogen atoms and one large negatively charged oxygen atom. When the hydrogens bind to the oxygen, it creates an asymmetrical molecule with positive charge on one side and negative charge on the other side (Figure 1). This charge differential is called polarity and dictates how water interacts with other molecules.
Figure 1: Water Chemistry. Water molecules are made of two hydrogens and one oxygen. These atoms are of different sizes and charges, which creates the asymmetry in the molecular structure and leads to strong bonds between water and other polar molecules, including water itself.
Water is the “Universal Solvent”
As a polar molecule, water interacts best with other polar molecules, such as itself. This is because of the phenomenon wherein opposite charges attract one another: because each individual water molecule has both a negative portion and a positive portion, each side is attracted to molecules of the opposite charge. This attraction allows water to form relatively strong connections, called bonds, with other polar molecules around it, including other water molecules. In this case, the positive hydrogen of one water molecule will bond with the negative oxygen of the adjacent molecule, whose own hydrogens are attracted to the next oxygen, and so on (Figure 1). Importantly, this bonding makes water molecules stick together in a property called
cohesion. The cohesion of water molecules helps plants take up water at their roots. Cohesion also contributes to water’s high boiling point, which helps animals
regulate body temperature.
by Molly Sargen
figures by Daniel Utter
Furthermore, since most biological molecules have some electrical asymmetry, they too are polar and water molecules can form bonds with and surround both their positive and negative regions. In the act of surrounding the polar molecules of another substance, water wriggles its way into all the nooks and crannies between molecules, effectively breaking it apart are dissolving it. This is what happens when you put sugar crystals into water: both water and sugar are polar, allowing individual water molecules to surround individual sugar molecules, breaking apart the sugar and dissolving it. Similar to polarity, some molecules are made of ions, or oppositely charged particles. Water breaks apart these ionic molecules as well by interacting with both the positively and negatively charged particles. This is what happens when you put salt in water, because salt is composed of sodium and chloride ions.
Water’s extensive capability to dissolve a variety of molecules has earned it the designation of “universal solvent,” and it is this ability that makes water such an invaluable life-sustaining force. On a biological level, water’s role as a solvent helps cells transport and use substances like oxygen or nutrients. Water-based solutions like blood help carry molecules to the necessary locations. Thus, water’s role as a solvent facilitates the transport of molecules like oxygen for respiration and has a major impact on the ability of
drugs to
reach their targets in the body.
....
continues. Biological Roles of Water: Why is water necessary for life? - Science in the News
That was only the first thing...
The list of helpful attributes is extensive, and I'll just pick a few at random:
Water plays another key role in the biochemistry of life: bending enzymes. Enzymes are proteins that catalyze chemical reactions, making them occur much faster than they otherwise would. To do their handiwork, enzymes must take on a specific three-dimensional shape. Never mind how, but it is water molecules that facilitate this.
...
... In fact, despite its ubiquity and molecular simplicity, H2O is abnormal in the extreme.
For starters, while other substances form liquids, precious few do so under the conditions of temperature and pressure that prevail on our planet's surface. In fact, next to mercury and liquid ammonia, water is our only naturally occurring inorganic liquid, the only one not arising from organic growth. It is also the only chemical compound that occurs naturally on Earth's surface in all three physical states: solid, liquid, and gas. Good thing, otherwise the hydrological cycle that most living things rely on to ferry water from the oceans to the land and back again would not exist. As science journalist Philip Ball writes in his informative book Life's Matrix: A Biography of Water, "This cycle of evaporation and condensation has come to seem so perfectly natural that we never think to remark on why no other substances display such transformations."
Waterless life
Could life as we
don't know it have gotten a start without water? Some planetary scientists have suggested that on certain very cold planetary bodies liquid ammonia might serve in place of water to incubate life. But even though it's the most common non-aqueous solvent, liquid ammonia would seem to have several other things going against it as a medium for life. Its liquid range is small, only about 30 degrees. Also, when it freezes, it sinks, and we know what that would do.
Some have suggested that oceans of methane or other hydrocarbons on places like Saturn's moon Titan could also serve the purpose. But, again, we're talking temperatures so low that chemical reactions as we know them could only proceed at a glacial pace. "At minus 150 degrees," says Bada, "most of the reactions that we think about in terms of being important in the origin of life probably wouldn't take place over the entire age of the solar system." Moreover, compounds like amino acids and DNA would not be soluble in these other liquids. "They would just be globs of gunk," Bada says."
Everybody knows that water is necessary for life, at least as we know it. But just why exactly?
www.pbs.org
Ok, let me just try to get a more broad summary that is brief....
This looks useful as a summary or reminder:
ADVERTISEMENTS: 1. Water is highly cohesive and adhesive: Because of hydrogen bonds, water molecules develop strong intermolecular attraction between them. This is called cohesion. When water form hydrogen bonds with other substance, the attraction is called adhesion. Due to cohesion and...
www.biologydiscussion.com