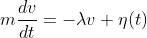- May 15, 2020
- 1,258
- 365
- Country
- United States
- Faith
- Christian
- Marital Status
- Private
I think a liquid is too dense to even consider B an outside possibility. A particle will have surface interactions with the liquid molecules that would prevent straightline motion.
If the 'particle' were itself an atom, and the fluid were a gas, then we might be in B territory.
Liquids have a wide range of densities & viscosities, so it may depend. It might also depend on the mass of the particle versus the forces applied by colliding particles, but fair enough. Would the motion of the particle in a dense, viscous liquid be more the "jiggling" that originally prompted curiosity about Brownian motion, or would it actually walk, just not in a straight line?
I like the possibility that one could derive conditions under which the particle wouldn't walk at all, but only jiggle in place. But it seems that if the particle can walk, then any path is possible. Or maybe I'm missing something in what you said.
Upvote
0